How To Get In Covid Vaccine Trial
According to guidance issued by the FDA in June 2020 in the event of a COVID-19 vaccine being judged safe and effective there may be a case for unblinding trials and offering the vaccine. COVID-19 vaccine side effects may last about a.
.png)
Covid19 Vaccine Wits University
In a first for vaccine development two COVID-19 vaccines were created evaluated and authorized for emergency use in under a year.
How to get in covid vaccine trial. The CDC continues to closely monitor these vaccines for safety and efficacy. Each vaccine group will enroll about 25 people ages 18 through 55 years and approximately 25 people age 56 years and older. And globally its unknown how much protection they get from vaccination.
How COVID-19 vaccines are being tested. Johnson and Johnson is known for its longstanding commitmentand proven track recordwhen it comes to fighting emerging epidemics. The UVM Medical Center is one of 80 sites in the country tasked with enrolling 30000 volunteers to get.
There you can begin the screening process to find out if. Top free images vectors for How to get in covid vaccine trial in png vector file black and white logo clipart cartoon and transparent. When she came back for the second dose in September she began to experience distressing symptoms.
You can join any of the government-sponsored coronavirus vaccine trials by going to the CoVPN website. Twelve to 20 weeks following their initial vaccination regimen participants will receive a single booster dose of the Moderna COVID-19 vaccine as part of the trial. Developing a vaccine is a top priority for the government FDA and other regulatory bodies.
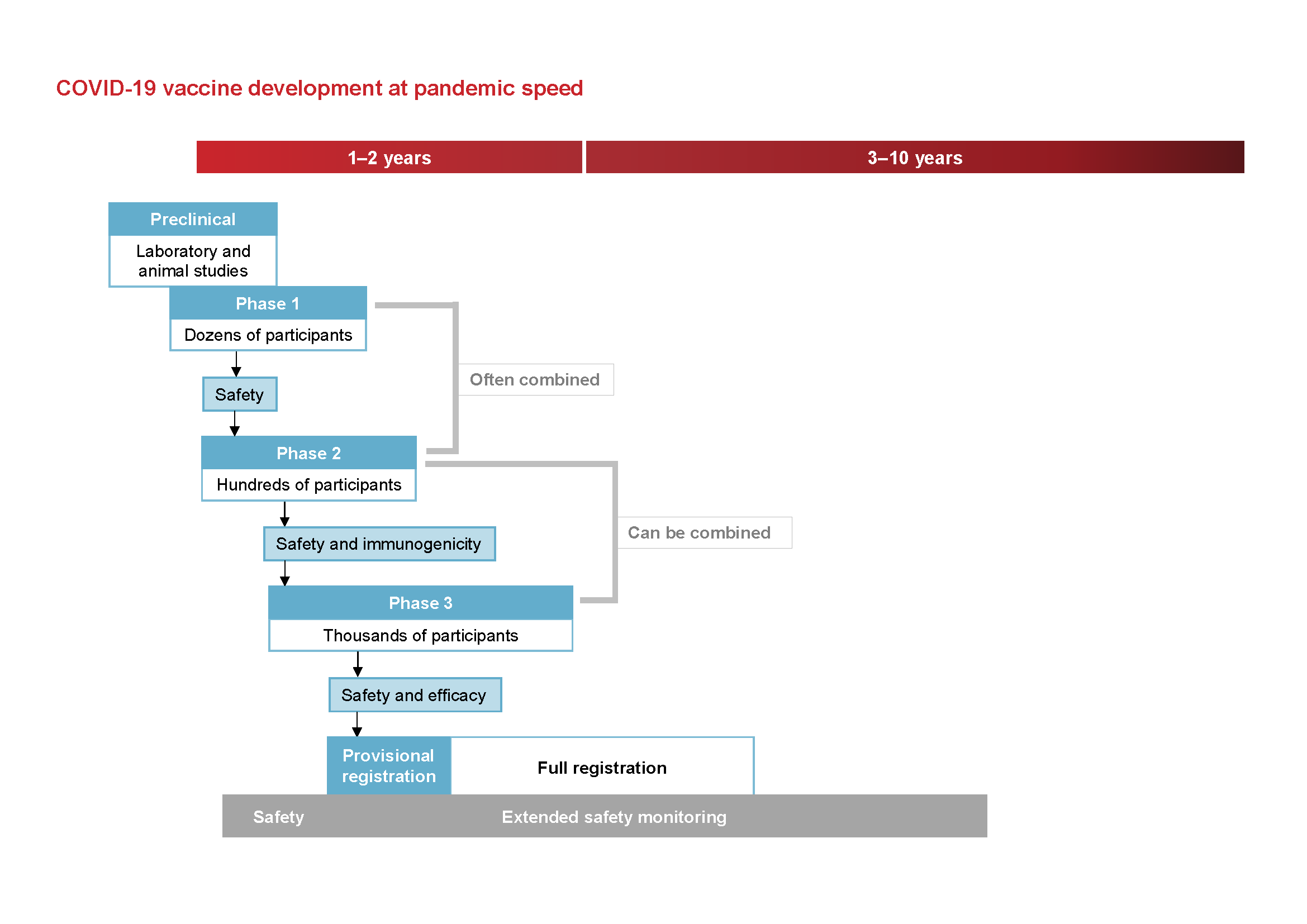
Different types of COVID-19 vaccines have been authorized in the US. Despite the fast timeline these vaccines went through the appropriate clinical trials just like other vaccines before. For example all COVID-19 vaccine clinical trial phases were planned at once to prevent the delay that can usually occur.
Before a vaccine is registered for use it is tested extensively during development and then in thousands of people. Testing first begins with laboratory research then animal studies and finally human clinical trials. However if it has not been controlled yet they cannot get a jab.
How the clinical trial works. Now its time to find out if it works to protect people from the coronavirus. The nurse volunteered to participate in Pfizers COVID-19 vaccine trial in August.
A Phase 3 randomized placebo-controlled observer-blind clinical trial to evaluate the efficacy safety and immunogenicity of the Moderna COVID19 Vaccine in participants 18 years of age and older is ongoing in the United States NCT04470427. Its Ebola vaccine was approved in early July by the European Commission and even in the midst of the COVID-19 pandemic the Janssen Pharmaceutical Companies of Johnson Johnson are still moving forward with its research into. Experts urge pregnant people to get vaccinated as new data show COVID-19 drastically increases the risks of death and pre-term birth.
Some people notice side effects of the COVID-19 vaccine such as pain or swelling where they got the vaccine. On this basis Iris encouraged autoimmune survivors who are in stable and healthy condition to get the Covid-19 vaccine immediately at the vaccination center. Kids between 12 and 16 have been eligible to get the Pfizer shot since May The trial at Rutgers began mid-June and has enrolled about 65 kids so far.
You may also get fever muscle aches chills fatigue headaches or a combination of these symptoms. The FDA has authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine to prevent COVID-19 in individuals 12 years of age and older under an Emergency Use Authorization EUA The FDA also confirms in their fact sheet that the Pfizer jab alongside all other Covid jabs is still in clinical trials Serious and unexpected side. There is no specific vaccine for people with autoimmune disease to date.
Because people with compromised immune systems were largely excluded from the Covid-19 vaccine trials in the US. To speed along the review of vaccine efficacy data a number of administrative changes were made that prioritized COVID vaccine work. This is the KidCOVE trial which is testing the Moderna mRNA COVID-19 vaccine in kids from 6 months up until just before they turn 12.
Learn how they work and what other vaccines are in phase 3 clinical trials. Pfizers vaccine trials for children under 12 are taking place at 101 sites around the world. Overview of what you should know about the different COVID-19 vaccines including vaccine types and how they work to provide protection against COVID.

Covid 19 Vaccine Trials To Begin In Israel Sheba Medical Center
Clinical Trials Nih Covid 19 Research

Pfizer Vaccine Results Are Promising But Lack Of Data Very Concerning Experts Say

Https Static Dw Com Image 58866702 7 Png
Moderna Covid Vaccine Has 94 Efficacy Final Results Confirm Coronavirus The Guardian
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center

News First Children S Covid 19 Vaccine Trial Open Nihr
Covid Vaccine Trials Were A Triumph Now We Need A Similar System For Antibiotics
What Is Mrna How Pfizer And Moderna Tapped New Tech To Make Coronavirus Vaccines

Valneva Vaccine Trial Phase Three University Of Southampton
Could Employers And States Mandate Covid 19 Vaccinations Here S What The Courts Have Ruled

A Covid 19 Vaccine May Come Soon Will The Blistering Pace Backfire Science News

Covid 19 Vaccine Trial Shifa International Hospital
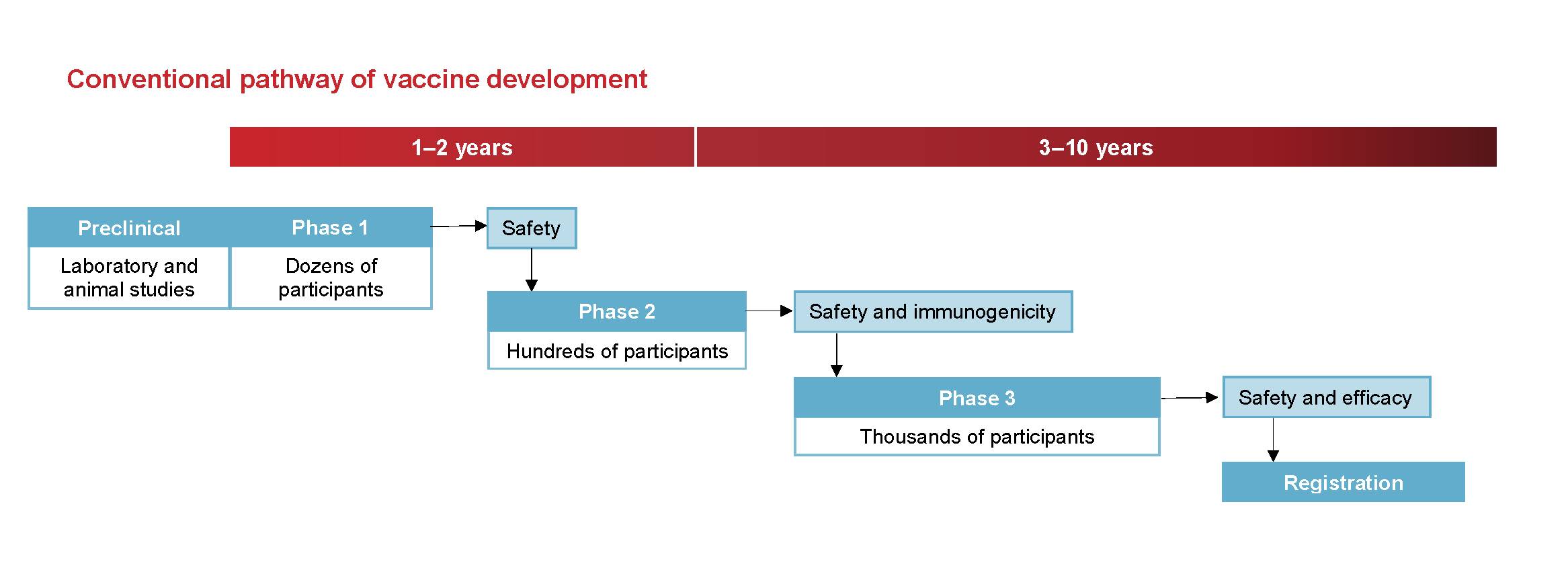
Phases Of Clinical Trials Ncirs
Phases Of Clinical Trials Ncirs

Https Static Dw Com Image 56382116 7 Png

Covid 19 Clinical Trials Michigan Medicine

Oxford Astrazeneca Will Test Covid Vaccine On Children The Washington Post

Covid Vaccines From Novavax And Medicago Could Debut Soon Shots Health News Npr
Post a Comment for "How To Get In Covid Vaccine Trial"